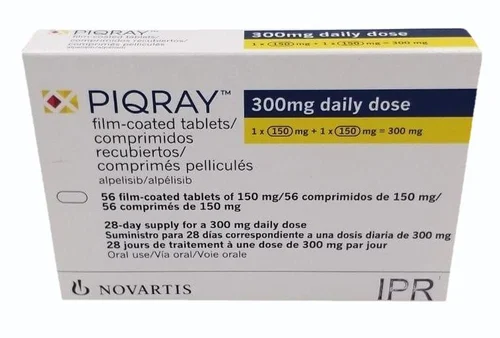
Buy Piqray (alpelisib) Online
Piqray, with the active ingredient Alpelisib, is a groundbreaking medication designed to target and treat specific types of breast cancer. Manufactured by Novartis, Piqray represents a significant advancement in cancer therapy, offering hope and improved outcomes for patients battling this disease.
Key Features:
- Targeted Therapy: Piqray (Alpelisib) is specifically formulated to inhibit the PI3K pathway, a crucial signaling pathway in many cancers, including breast cancer. By targeting this pathway, Piqray helps to slow or stop the growth of cancer cells.
- Effective Dosage: Available in an oral form, Piqray provides a convenient and effective method of administration, allowing patients to maintain their treatment regimen with ease.
- Trusted Manufacturer: Produced by Novartis, a global leader in pharmaceuticals, Piqray adheres to the highest standards of quality, safety, and efficacy, ensuring reliable treatment for breast cancer patients.
Indications Piqray (alpelisib) Online:
Piqray (Alpelisib) is indicated for the treatment of:
- Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer: Piqray is used in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen.
Usage:
- Form: Oral tablets
- Administration: Piqray should be taken orally, as directed by a qualified healthcare professional. It is typically prescribed in combination with another medication, such as fulvestrant.
- Dosage: The dosage and treatment plan for Piqray will vary based on the patient’s medical condition, response to treatment, and other factors. Follow the prescribed dosage regimen strictly and consult your healthcare provider for any questions or concerns regarding your treatment.
Safety Information:
- Precautions: Before starting Piqray, inform your healthcare provider about your medical history, especially any history of diabetes, liver disease, or any other medical conditions. Regular monitoring of blood sugar levels and liver function tests may be required during treatment.
- Potential Side Effects: Common side effects of Piqray include high blood sugar (hyperglycemia), nausea, diarrhea, decreased appetite, rash, and fatigue. Serious side effects may include severe hyperglycemia, severe skin reactions, and pneumonitis. Contact your healthcare provider if you experience any severe or persistent side effects.
- Drug Interactions: Inform your healthcare provider about all the medications you are taking, including prescription, over-the-counter drugs, and supplements, as Piqray may interact with other medications.
Experience the Hope of Piqray:
With Piqray, Novartis offers an innovative and effective treatment option for patients with advanced breast cancer. Trust in the advanced science and proven efficacy of Piqray to provide targeted therapy, offering hope and improved outcomes for those battling this challenging disease. Embrace the future of breast cancer treatment with Piqray and take a significant step towards better health and well-being.



Reviews
There are no reviews yet.